© elfruler 2019-2020
Click here for an Introduction to this page.
Photos here are from the 2019 breeding season in a Bald Eagle’s nest in Bluff City, TN, broadcast live by East Tennessee State University, and are used by permission. Click here for link to the live cam. The focus here is on the elder of two eaglets, BC14. Many thanks to Michelle France and Donna Young for helping to collect the screen captures and tell the story.
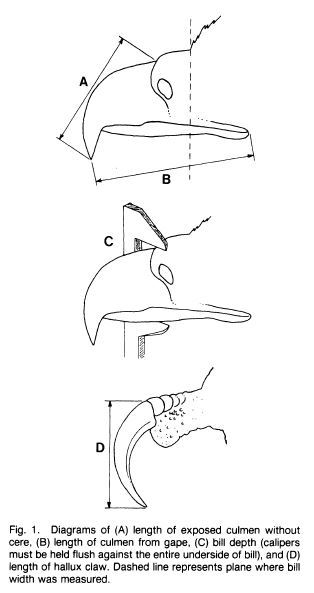
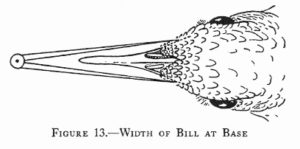
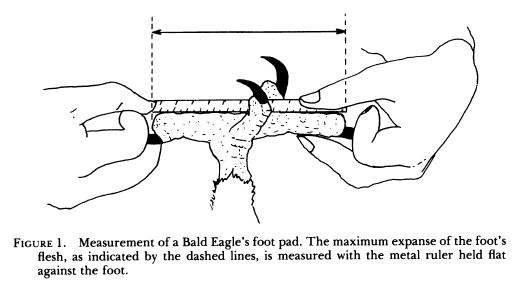
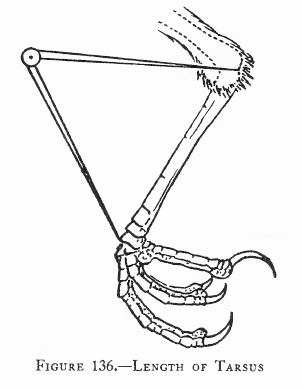
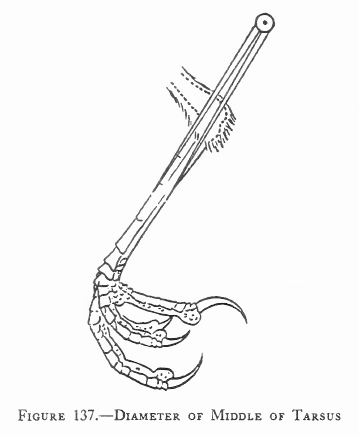
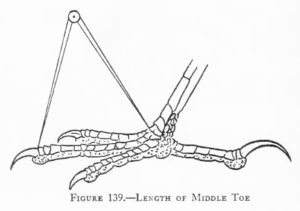
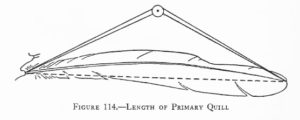
Measurements are derived or calculated mainly from Bortolotti 1984a, 1984c, and 1984d, and Gerrard and Bortolotti 1988 (Click here for References). Numbers given here are in the ball park but will vary from one eaglet to another. % indicates the proportion a particular measurement bears to its value at the juvenile’s full size (Click here for information about taking measurements.)
BC14 hatched at 10:32 a.m. on 3/11/19. The cam provided a rare bird’s-eye (pun intended) view of how an eaglet uncurls itself from inside the egg in the first few seconds of hatching.
I’ve slowed the stream to 10% of normal speed and added arrows to indicate the back, head, left wing, right wing, beak, tail, legs and feet, egg tooth (yes! the egg tooth!), umbilicus, and receding yolk sac of the hatchling. The eaglet has its back to us and its head is down, tail up.
In the weekly galleries below, click on photos for larger views and to scroll through images. The numbering of Days refers to the age of BC14; Day 0 is Hatch Day, Day 1 is 24 hours after hatch, etc.




The eaglet is hatched with pink skin covered by a thin layer of light gray natal down. The beak and cere are gray, facial skin is dark, legs and feet are pinkish-cream-colored, eye ring is dark and protruding. The eaglet is weak, with limited mobility, balance, and vision. First feeding can occur within about 2 ½ hours.
| HEIGHT | WEIGHT | BEAK LENGTH | FOOT PAD | ||
|---|---|---|---|---|---|
| Day 0 |








Skin color changes from pink to bluish-gray, feet and legs are cream-colored; cere turns from pale gray to pale yellow. Steadily gains strength and balance through the week, gaining ability to take food, “swim” on belly with wings and legs, escape from nest cup. Eyes focusing better, seeking out and imprinting on parents. Sibling competition begins. Natal down and egg tooth remain through the week. First PS can occur about 12 hours after first feeding.
| Day 6 |








Skin around eyes becomes lighter gray. Supraorbital ridge more prominent by mid-week. Egg tooth disappears. Cere turns gray, rictus of mouth pale yellow. Feet are beginning to grow rapidly; talons are beige and growing. Second down appears as early as Day 6. Juvenal contour feathers (pin feathers) start to emerge on wings, back, and legs by week’s end; male’s remiges emerge earlier than female’s. (The contour feathers emerge from the natal down follicles and push the natal down out as they grow.) Facial bristle feathers emerging around eyes and beak. Reaching out for food more actively; backing up to nest edge to slice; walking sturdily on hocks; preening, stretching, flapping, and scooting as far out as the nest rails.
| Day 13 |








Growth spurt begins and size differences between male and female develop, especially in weight, beak length, foot pad, and 8th primary feather. Females are larger, but males grow earlier and more quickly. By mid-week, female is gaining 70-180g per day, male 80-150g per day. Beak and feet growing rapidly. Talons turn from beige to black. Second down thickens, approaching ability to thermoregulate. Some second down is growing on the front of the upper region of the tarsometatarsi. Wing primary and covert feathers lengthen, pushing out natal down at the tips; primaries grow a little over 7mm per day. Contour feathers emerge on back, shoulders, legs, and breast. Rectrices start to emerge by mid-week. Beginning to stand on toes briefly. Stretching and rousing, pellet casting, resting on the rails.
| Day 20 | (F 57%, M 61%) |








Growth spurt continues. Remiges are increasingly measurable. Rictus of mouth is yellow. Second down covers entire body except on top of head where natal down layer is still prominent (resulting in that famous “mohawk” look). Head, neck, and side contour feathers coming in. Pecking at food, grasping and playing with nesting materials, wobbly toe-walking by the end of the week. Sibling competition transitions into play.
| Day 27 | M 115mm (87%) | ||||
| Day 27-33 |








Energy demands for metabolism and growth peak by Day 30, then weight gain tapers off. Feet nearly full size by week’s end and are turning yellow. Stretching, flapping, preening, and more confident toe-standing and -walking. Standing on nest twigs as if on a branch, practicing holding with toes and talons. Parents hold food further away to encourage reaching; eaglet may lunge for food and attempt to tear off bites with beak; hasn’t yet mastered the skill of holding food down with feet.
| Day 34 | M 3000g (72%) | M 27mm (87%) | M 129 (98%) | |
| Day 32-38 | M 110-116mm |








At week’s end the eaglet is about 3/4 of full weight. Feet are nearly full grown. Toes and tarsometatarsi fully grown (making banding possible). Legs, feet, lores, and rictus of mouth are yellow. Stretching and flapping, standing securely, grabbing and mantling food as it is delivered, tearing food more effectively. Grasping and playing with nest materials with talons and beak. Vocalizations transitioning from chirps to persistent chittering and loud “squees,” especially at parental visits and food deliveries.
| Day 41 | M 3300g (73%) | M 29mm (90%) | M 130 (98%) | |
| Day 37-43 | M 146-152mm |








From Days 40-45 growth of beak and feet slows; feet and legs will be fully grown by Day 50. Contour feathers on front of the upper region of the tarsometatarsi emerging. Wing flapping becoming more vigorous, flap-hopping higher. Standing on the rails to slice. Long stretches of standing and looking out, or sleeping on the rails.
| Day 48 | M 3500g (88%) | M 30mm (92%) | M 132mm (100%) | |
| Day 42-48 | M 181-187mm |








Contour feathers on sides and belly filling in. Whitish sheaths still visible at bases of remiges and upper- and underwing coverts. Confident standing. More effective self-feeding, but still relies on parents for most feedings; grabbing, stealing, and mantling food. Vigorous flapping, lifting off, enjoying the wind. May begin branching, perhaps with 1-2 flaps, often by stepping.
| Day 55 | M 3700g (92%) | M 30mm (93%) | |
| Day 47-53 | M 217-233mm |








After about Day 60 growth tapers off except beak, hallux claw, and flight feathers. Some remnants of sheaths at bases of wing coverts. Juvenal body feathers nearly complete except on wings and tail; contour feathers on flanks still filling in, as well as on the upper region of the tarsometatarsi. Axillary feathers (wingpits) mostly white. Aggressively grabbing and attempting to steal food from parents and siblings. Flapping results in hovering in mid-air for several seconds. Siblings watch, mimic, and play with each other. Branching likely.
| Day 62 | M 3850g (96%) | M 30mm (94%) | ||
| Day 52-58 | M 253-259mm |
|||
| Day 57-63 | M 288-294mm |
Lower leg feathers are thickening. Aggressive food grabbing, stealing, and mantling. Confident one-foot perching and preening on branches. Vigorous flapping and long hovers. Sometimes stumbles when landing, learning to use wings to regain balance. Fledging is possible from Weeks 10-13. Males usually fledge 3-4 days before females.
| Day 62-68 | M 324-330mm |
Growth of primaries slows after 72 days. Branching more confidently, learning to perch, move around, and use wings for balance on branches. Fledging can occur suddenly and without warning, although eaglet may look intently at nearby branches and appears to evaluate suitable landing spots. First landing is usually awkward, and eaglet may end up on the ground.
| Day 67-73 | M 360-366mm |
By Day 80 primaries have reached 80% and rectrices 84% of their full length (achieved in the second winter). Male primaries growth tapers off, but female primaries continue to grow after fledge. Juvenal feathers will be longer than those of mature adults and will become shorter with each molt until year 5. Beak and hallux talon not yet fully grown at fledge (they will reach full size by the second or third winter).
| Day 80 | M 50mm |
BC14 fledged unintentionally on Day 81, 5/31/19, but the eaglet was ready. The branch on which it was perched broke and the eaglet fell but quickly recovered and flew strongly in the direction of trees across the creek.
The new fledgling juvenile eagle returned to the nest 3 days later and visited several times before dispersing from the area for good. Its sibling, BC15, fledged 8 days after BC14.
PERSONAL NOTE: In my opinion the 2 eaglets at this nest in 2019 are of the same gender, either female-female or male-male (it is impossible to know which). The younger may appear to be slightly smaller, but according to Bortolotti (1986a, 1986b), a younger sibling is almost always slightly smaller than an elder of the same gender. A male develops earlier and more quickly, but a female eventually is noticeably larger, especially so if she is the elder. (Male-female broods are quite rare.) It is quite difficult to ascertain relative size because of the camera angle and lack of perspective, but at fledge I could not see a significant size difference between BC14 and BC15.

























You must be logged in to post a comment.